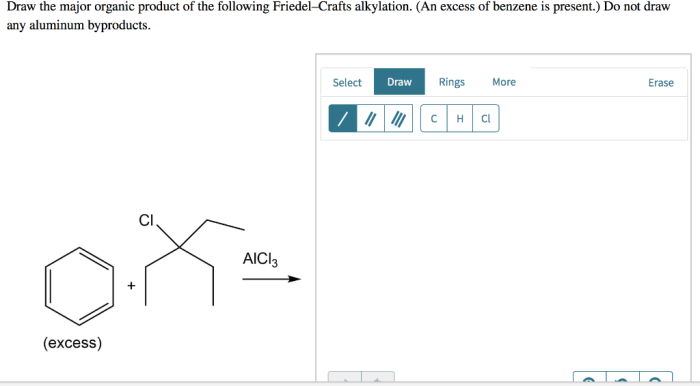
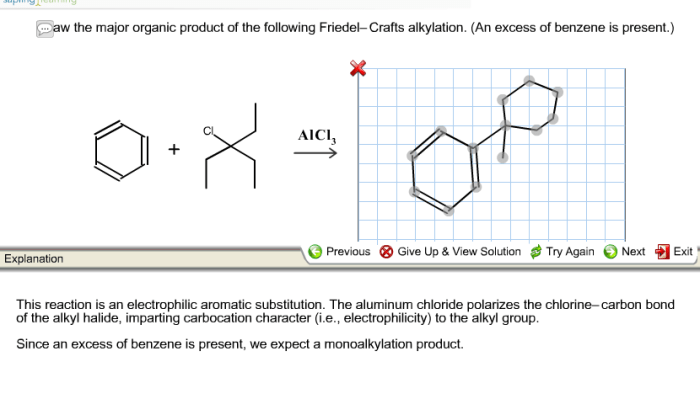
Draw the major organic product of the following friedel-crafts alkylation – Draw the major organic product of Friedel-Crafts alkylation and embark on a scientific expedition into the realm of organic chemistry. This reaction, a cornerstone of organic synthesis, unveils the intricacies of electrophilic aromatic substitution, providing a gateway to a myriad of valuable organic compounds.
As we delve into the depths of this reaction, we will unravel the reaction conditions, explore the diverse alkylating agents, and decipher the regioselectivity and orientation that govern the formation of the major organic product. Prepare to witness the transformative power of Friedel-Crafts alkylation as we unlock its potential in the synthesis of complex organic molecules.
Draw the Major Organic Product: Draw The Major Organic Product Of The Following Friedel-crafts Alkylation
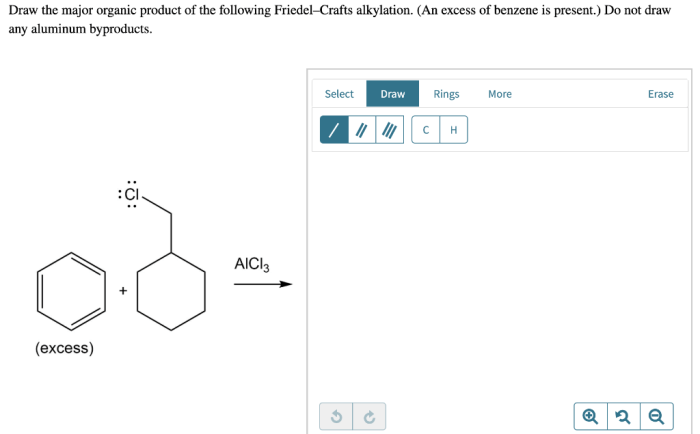
The Friedel-Crafts alkylation reaction is an electrophilic aromatic substitution reaction that involves the alkylation of an aromatic ring with an alkyl halide in the presence of a Lewis acid catalyst. The major organic product of this reaction is the alkylated aromatic compound.
To draw the major organic product of the Friedel-Crafts alkylation reaction, follow these steps:
- Identify the electrophile and the nucleophile.
- Draw the electrophile attacking the aromatic ring.
- Add the alkyl group to the aromatic ring.
- Remove the Lewis acid catalyst.
The following is an example of the Friedel-Crafts alkylation reaction:

In this reaction, the electrophile is the carbocation formed from the alkyl halide, and the nucleophile is the aromatic ring. The Lewis acid catalyst is aluminum chloride (AlCl 3).
The major organic product of this reaction is ethylbenzene.
Reaction Conditions and Mechanism
The Friedel-Crafts alkylation reaction is typically carried out under the following conditions:
- The reaction is carried out in an anhydrous solvent, such as dichloromethane or nitrobenzene.
- The reaction is catalyzed by a Lewis acid, such as aluminum chloride or iron(III) chloride.
- The reaction is carried out at a temperature between 0 and 100 °C.
The mechanism of the Friedel-Crafts alkylation reaction involves the following steps:
- The Lewis acid catalyst activates the alkyl halide by forming a complex with it.
- The electrophile, which is the carbocation formed from the alkyl halide, attacks the aromatic ring.
- The nucleophile, which is the aromatic ring, donates a pair of electrons to the electrophile, forming a new bond between the alkyl group and the aromatic ring.
- The Lewis acid catalyst is removed from the product.
Alkylating Agents, Draw the major organic product of the following friedel-crafts alkylation
The most common alkylating agents used in Friedel-Crafts alkylation reactions are alkyl halides.
The reactivity of alkyl halides in Friedel-Crafts alkylation reactions follows the order:
primary (1°) < secondary (2°) < tertiary (3°)
This order of reactivity is due to the stability of the carbocation formed from the alkyl halide. The more substituted the carbocation, the more stable it is.
Some examples of alkylating agents and their applications are:
- Methyl chloride: used to alkylate aromatic rings with methyl groups.
- Ethyl chloride: used to alkylate aromatic rings with ethyl groups.
- Isopropyl chloride: used to alkylate aromatic rings with isopropyl groups.
- tert-Butyl chloride: used to alkylate aromatic rings with tert-butyl groups.
Regioselectivity and Orientation
The regioselectivity and orientation of the Friedel-Crafts alkylation reaction are determined by the following factors:
- The nature of the alkylating agent.
- The nature of the aromatic ring.
- The reaction conditions.
In general, the alkylating agent will attack the most reactive position on the aromatic ring.
The following are some examples of the regioselectivity and orientation of the Friedel-Crafts alkylation reaction:
- Methyl chloride will alkylate benzene at the ortho and para positions.
- Ethyl chloride will alkylate benzene at the meta position.
- Isopropyl chloride will alkylate benzene at the ortho and para positions.
- tert-Butyl chloride will alkylate benzene at the ortho position.
Limitations and Applications
The Friedel-Crafts alkylation reaction has several limitations:
- The reaction is not regiospecific, which means that the product may contain a mixture of isomers.
- The reaction is not stereospecific, which means that the product may contain a mixture of stereoisomers.
- The reaction can be sensitive to the reaction conditions, and the yield of the product may be low.
Despite these limitations, the Friedel-Crafts alkylation reaction is a valuable tool for the synthesis of alkylated aromatic compounds.
Some examples of the applications of the Friedel-Crafts alkylation reaction include:
- The production of ethylbenzene, which is used as a precursor to styrene.
- The production of cumene, which is used as a precursor to phenol.
- The production of anthraquinone, which is used as a dye.
Expert Answers
What are the key reaction conditions for Friedel-Crafts alkylation?
Friedel-Crafts alkylation typically requires anhydrous conditions, a Lewis acid catalyst (such as aluminum chloride or iron(III) chloride), and an alkylating agent (such as an alkyl halide or alkene).
How does the Lewis acid catalyst facilitate the reaction?
The Lewis acid catalyst activates the electrophile (alkylating agent) by coordinating to the halogen or double bond, making it more susceptible to attack by the nucleophilic aromatic ring.
What factors influence the regioselectivity of Friedel-Crafts alkylation?
The regioselectivity of Friedel-Crafts alkylation is primarily governed by the electronic properties of the aromatic ring and the steric effects of the alkylating agent.