Sketch a qualitative energy diagram for the dissolution of nh4cl – Delving into the intricacies of “Sketching a Qualitative Energy Diagram for the Dissolution of NH4Cl,” this exploration embarks on an enlightening journey through the fundamental concepts of chemistry, thermodynamics, and the behavior of matter at the molecular level. As we unravel the complexities of this process, we will gain a deeper understanding of the energetic landscape that governs the dissolution of NH4Cl, shedding light on its significance in various chemical and industrial applications.
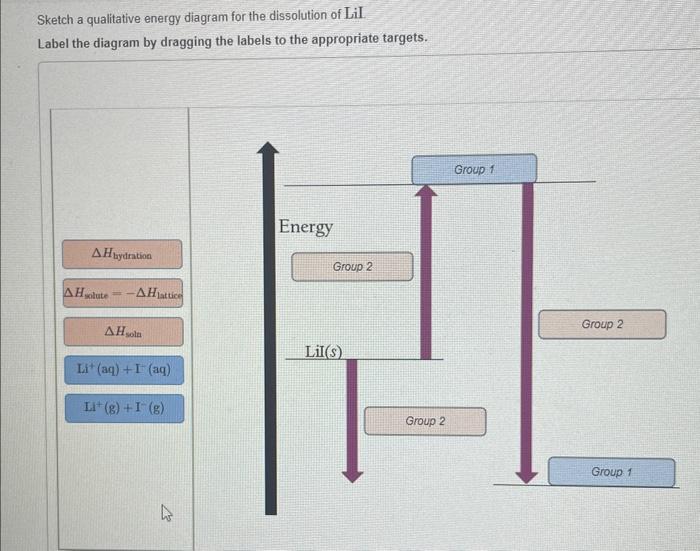
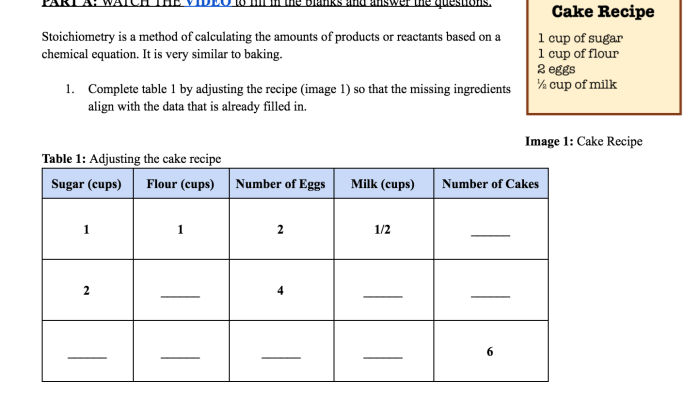
The dissolution of NH4Cl, a ubiquitous process in chemistry, involves the breaking apart of its crystal lattice and the solvation of its constituent ions in water. This intricate process is accompanied by a myriad of energy changes that can be effectively depicted using a qualitative energy diagram.
By constructing such a diagram, we can visualize the energetic barriers and driving forces that shape the dissolution process, providing valuable insights into its spontaneity, kinetics, and potential applications.
Qualitative Energy Diagram for the Dissolution of NH4Cl: Sketch A Qualitative Energy Diagram For The Dissolution Of Nh4cl
A qualitative energy diagram provides a graphical representation of the energy changes that occur during a chemical reaction. It helps visualize the energy barrier that must be overcome for the reaction to proceed and the overall energy change associated with the process.
In this article, we will sketch a qualitative energy diagram for the dissolution of NH4Cl in water.
Energy Changes During Dissolution
The dissolution of NH4Cl in water is an endothermic process, meaning that it requires energy input. This energy is used to overcome the attractive forces between the NH4+ and Cl- ions in the solid NH4Cl lattice and to form new interactions between these ions and water molecules.
- Lattice energy:The energy required to separate the NH4+ and Cl- ions from the crystal lattice.
- Hydration energy:The energy released when the ions interact with water molecules and become surrounded by a hydration shell.
The overall energy change during dissolution is the sum of the lattice energy and the hydration energy.
Sketching the Energy Diagram
To sketch the energy diagram, we plot the energy of the system on the y-axis and the reaction coordinate on the x-axis.
- The initial state is represented by the solid NH4Cl crystal, with a higher energy due to the strong lattice energy.
- The final state is represented by the hydrated ions in solution, with a lower energy due to the favorable hydration energy.
- The activation energy is the energy barrier that must be overcome for the reaction to proceed.
Factors Affecting the Energy Diagram, Sketch a qualitative energy diagram for the dissolution of nh4cl
- Temperature:Increasing temperature provides more energy to overcome the activation energy, leading to a faster dissolution rate.
- Concentration:Higher concentrations of NH4Cl increase the number of ions in solution, reducing the hydration energy and making the dissolution process less favorable.
- Catalysts:Catalysts can provide an alternative pathway for the reaction, lowering the activation energy and accelerating the dissolution rate.
Applications of the Energy Diagram
- Predicting the spontaneity and kinetics of the reaction.
- Designing experiments to optimize the dissolution process.
- Understanding the effects of different factors on the dissolution rate.
Question & Answer Hub
What is the significance of sketching a qualitative energy diagram for the dissolution of NH4Cl?
Sketching a qualitative energy diagram provides a visual representation of the energy changes that occur during the dissolution process, enabling us to understand the driving forces and barriers involved. It helps predict the spontaneity and kinetics of the reaction, optimize experimental conditions, and design efficient chemical systems.
How does lattice energy influence the energy diagram of NH4Cl dissolution?
Lattice energy represents the energy required to break apart the crystal lattice of NH4Cl. A higher lattice energy results in a higher activation energy for dissolution, making the process less spontaneous. The energy diagram reflects this by showing a steeper initial slope.
What is the role of hydration energy in the dissolution of NH4Cl?
Hydration energy is the energy released when ions are surrounded by water molecules. In the dissolution of NH4Cl, hydration energy contributes to the overall energy decrease, making the process more spontaneous. The energy diagram shows a decrease in energy as the ions become hydrated.