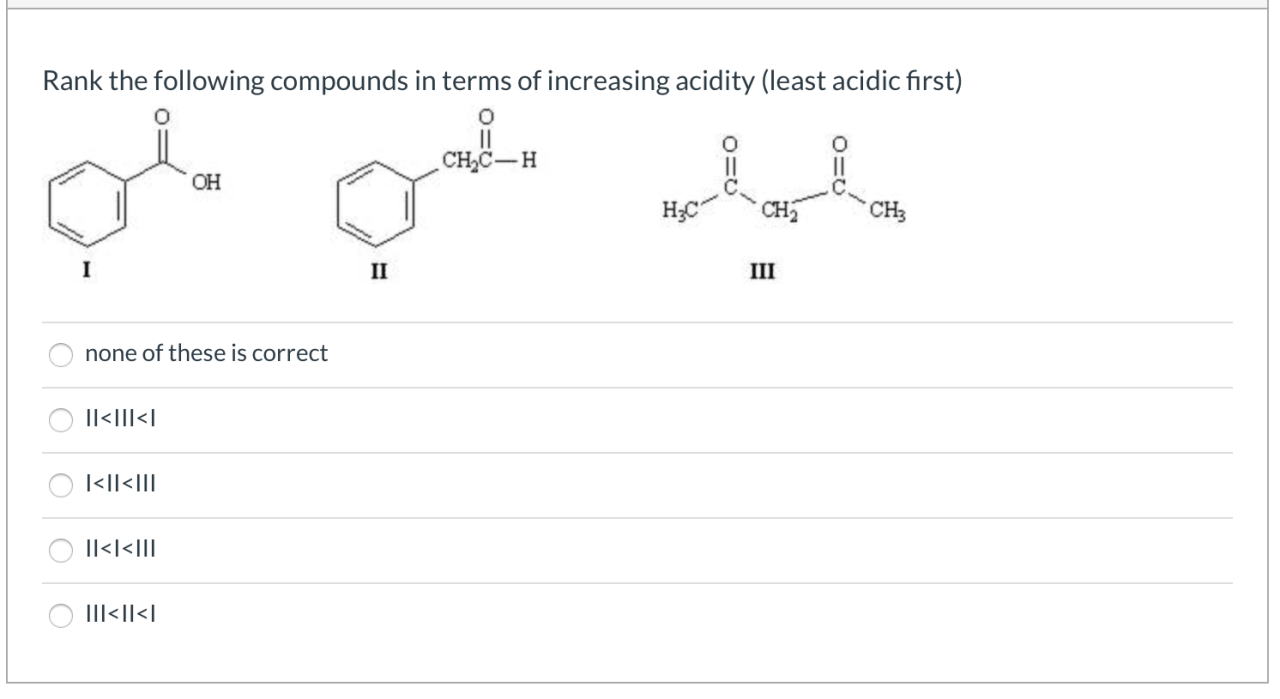
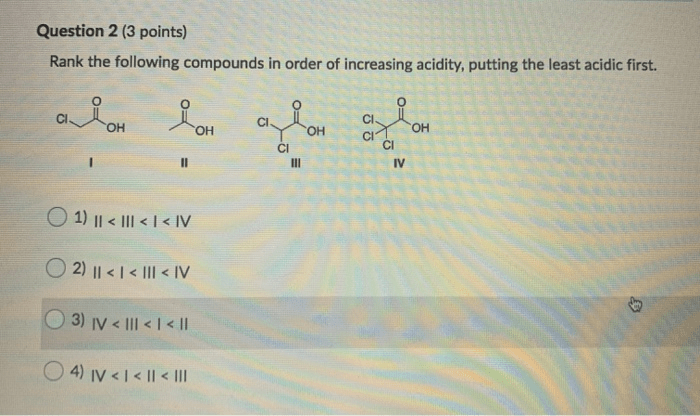
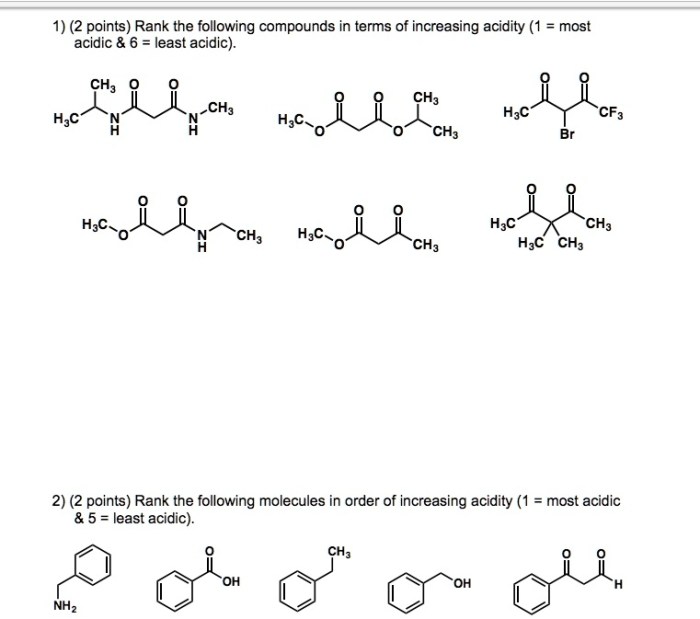
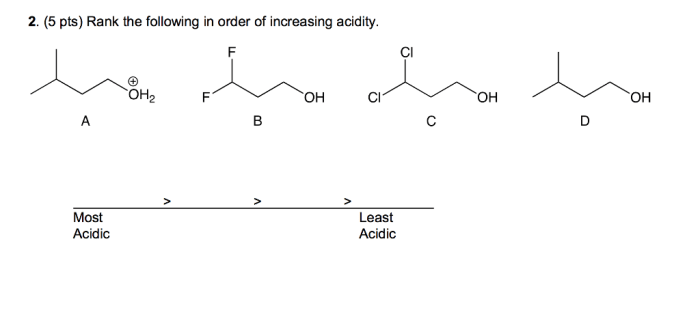
Rank the following compounds in terms of increasing acidity, a topic of paramount importance in chemistry, biology, and medicine. Understanding the acidity of compounds is crucial for comprehending their reactivity and behavior in various chemical and biological systems.
This discourse will delve into the concept of acidity, exploring the factors that influence it and the methods employed to rank compounds based on their acidic strength. Moreover, we will examine the practical applications of acidity ranking in diverse fields, highlighting its significance in scientific research and technological advancements.
1. Understanding Acidity
Acidity refers to the ability of a compound to donate a proton (H +ion) to another compound. The strength of an acid is measured by its acidity constant (K a), which is the equilibrium constant for the dissociation of the acid in water.
The higher the K avalue, the stronger the acid.
Factors that influence acidity include:
- Molecular structure
- Functional groups
- Hybridization
- Solvent effects
2. Ranking Compounds by Acidity
Compounds can be ranked by acidity using various methods, including:
- Kavalues: The most direct method, which provides a quantitative measure of acidity.
- pKavalues: The negative logarithm of the K avalue, which is often more convenient to use.
- Hammett acidity function:A measure of acidity in non-aqueous solvents.
Each method has its own strengths and limitations. K avalues are the most accurate measure of acidity, but they can be difficult to determine experimentally. pK avalues are easier to determine, but they are not as accurate as K avalues.
The Hammett acidity function is useful for measuring acidity in non-aqueous solvents, but it is not as general as the K aor pK avalues.
3. Example Compounds
| Compound | Ka | pKa | Ranking |
|---|---|---|---|
| Hydrochloric acid (HCl) | 1.0 x 10-1 | -1.0 | Most acidic |
| Acetic acid (CH3COOH) | 1.8 x 10-5 | 4.7 | |
| Water (H2O) | 1.0 x 10-14 | 14.0 | |
| Sodium hydroxide (NaOH) | 1.0 x 10-13 | 13.0 | Least acidic |
The compounds are ranked in increasing order of acidity, with hydrochloric acid being the most acidic and sodium hydroxide being the least acidic.
4. Factors Influencing Acidity
The acidity of a compound is influenced by several factors, including:
- Molecular structure:The arrangement of atoms in a molecule can affect its acidity. For example, compounds with more electronegative atoms tend to be more acidic.
- Functional groups:The presence of certain functional groups can make a compound more acidic. For example, carboxylic acids (COOH) and sulfonic acids (SO 3H) are both highly acidic.
- Hybridization:The hybridization of the atom that donates the proton can also affect acidity. For example, sp 3-hybridized atoms are more acidic than sp 2-hybridized atoms.
5. Applications of Acidity Ranking
Ranking compounds by acidity has many practical applications, including:
- Chemistry:Acidity is a key factor in many chemical reactions. For example, the acidity of a catalyst can affect the rate of a reaction.
- Biology:Acidity is important in many biological processes, such as enzyme catalysis and protein folding.
- Medicine:Acidity is a factor in the development and treatment of many diseases, such as ulcers and cancer.
Essential FAQs: Rank The Following Compounds In Terms Of Increasing Acidity
What is the significance of ranking compounds by acidity?
Ranking compounds by acidity allows scientists to predict their reactivity, solubility, and other properties. This information is essential for designing experiments, synthesizing new compounds, and understanding chemical processes in biological systems.
How can we experimentally determine the acidity of a compound?
The acidity of a compound can be determined experimentally using various methods, including pH measurement, titration, and conductometry. Each method has its advantages and limitations, and the choice of method depends on the specific compound and the desired accuracy.
What factors influence the acidity of a compound?
The acidity of a compound is influenced by several factors, including its molecular structure, functional groups, and hybridization. Compounds with more electronegative atoms, weaker bonds to hydrogen atoms, and greater resonance stabilization tend to be more acidic.