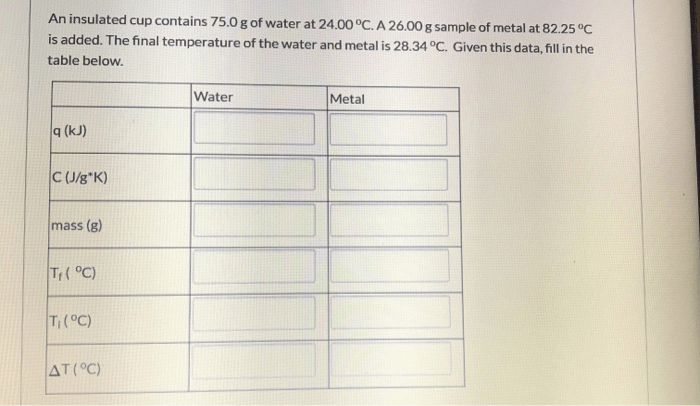
An insulated cup contains 75.0g of water – An insulated cup containing 75.0g of water serves as an ideal platform to delve into the captivating realm of thermal insulation and heat transfer. This comprehensive exploration unravels the intricate mechanisms that govern heat flow, shedding light on the crucial role of insulated cups in preserving temperature and conserving energy.
Delving into the heart of the matter, we will dissect the fundamental principles of thermal insulation, examining how it impedes heat transfer and maintains the integrity of the cup’s contents. Furthermore, we will meticulously analyze the various mechanisms of heat transfer, including conduction, convection, and radiation, and elucidate their interplay within the context of an insulated cup.
Thermal Insulation: An Insulated Cup Contains 75.0g Of Water

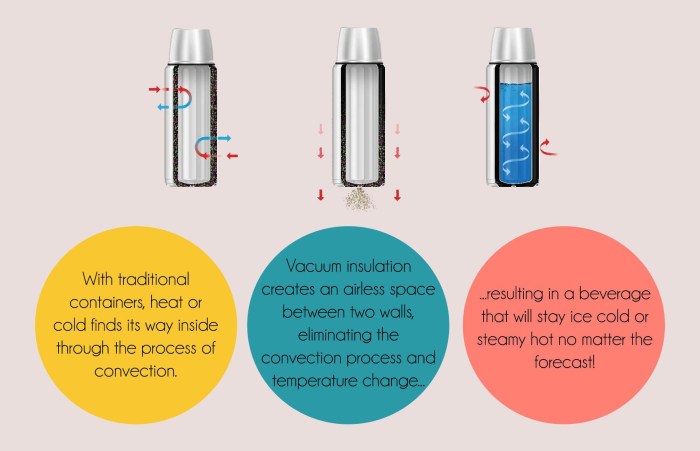
Thermal insulation is a material or technique that reduces the transfer of heat between two objects. It works by creating a barrier that impedes the flow of heat, thereby minimizing energy loss or gain.
In the context of an insulated cup, the cup itself acts as the thermal insulation. It is typically made of materials with low thermal conductivity, such as plastic, metal, or ceramic, which are poor conductors of heat.
Heat Transfer Mechanisms
Heat transfer occurs through three primary mechanisms: conduction, convection, and radiation.
- Conduction: Heat transfer through direct contact between two objects at different temperatures.
- Convection: Heat transfer through the movement of fluids (liquids or gases) from a hotter region to a cooler region.
- Radiation: Heat transfer through the emission and absorption of electromagnetic waves.
In the case of an insulated cup, conduction and convection are the primary heat transfer mechanisms. Conduction occurs through the cup’s walls, while convection occurs within the water inside the cup.
Water Properties
Water has a specific heat capacity of 4.18 J/g°C, which means that it takes 4.18 joules of energy to raise the temperature of 1 gram of water by 1 degree Celsius.
For the 75.0g of water in the insulated cup, the amount of heat required to raise the temperature by ΔT degrees Celsius is:
“`Q = m × c × ΔT“`
- Q: Heat energy (in joules)
- m: Mass of water (in grams)
- c: Specific heat capacity of water (4.18 J/g°C)
- ΔT: Temperature change (in degrees Celsius)
Heat Loss Calculations
The rate of heat loss from the insulated cup can be calculated using the following formula:
“`Q/t = h × A × (T cup
Tenv)
“`
- Q/t: Rate of heat loss (in joules per second)
- h: Convective heat transfer coefficient (in W/m 2K)
- A: Surface area of the cup (in square meters)
- T cup: Temperature of the cup (in Kelvin)
- T env: Temperature of the surrounding environment (in Kelvin)
Experimental Design
| Materials | Procedures | Data Collection |
|---|---|---|
| – Insulated cup- Water- Thermometer | 1. Fill the cup with water.2. Measure the initial temperature of the water.3. Place the cup in a controlled environment.4. Record the temperature of the water at regular intervals. | – Temperature of the water over time |
Data Analysis, An insulated cup contains 75.0g of water
The rate of heat loss can be determined by plotting the temperature of the water over time and calculating the slope of the line.
The slope of the line represents the change in temperature per unit time, which can be converted to the rate of heat loss using the formula:
“`Q/t = m × c × (ΔT/t)“`
- Q/t: Rate of heat loss (in joules per second)
- m: Mass of water (in grams)
- c: Specific heat capacity of water (4.18 J/g°C)
- ΔT/t: Slope of the line (change in temperature per unit time)
Applications and Implications
Understanding heat transfer in insulated cups has practical applications in various fields, including:
- Thermal insulation in buildings: Designing energy-efficient buildings by using insulated walls and roofs to minimize heat loss.
- Industrial processes: Optimizing industrial processes that involve heat transfer, such as cooling systems and heating equipment.
- Consumer products: Designing insulated containers and appliances to maintain the temperature of food and beverages.
FAQ Explained
What is the primary function of an insulated cup?
An insulated cup is designed to minimize heat transfer between its contents and the surrounding environment, effectively maintaining the temperature of the contents for extended periods.
How does an insulated cup prevent heat loss?
Insulated cups employ a combination of materials and design features, such as vacuum insulation or double walls with reflective coatings, to impede heat transfer through conduction, convection, and radiation.
What factors influence the rate of heat loss from an insulated cup?
The rate of heat loss is primarily determined by the temperature difference between the cup’s contents and the surroundings, the thermal conductivity of the cup’s materials, and the surface area of the cup exposed to the environment.